Vapor Pressure Of Water At Room Temperature Mmhg
Vapor pressure or vapour pressure in british english.
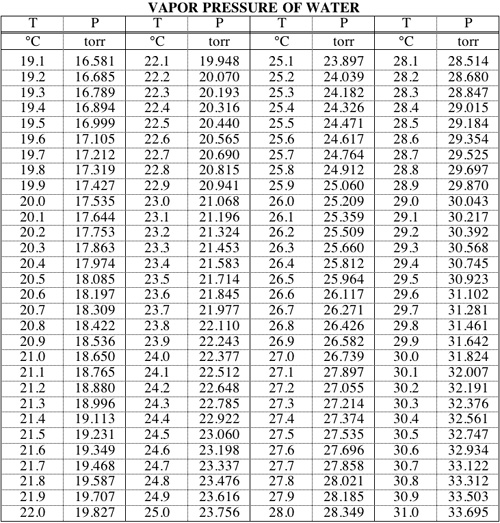
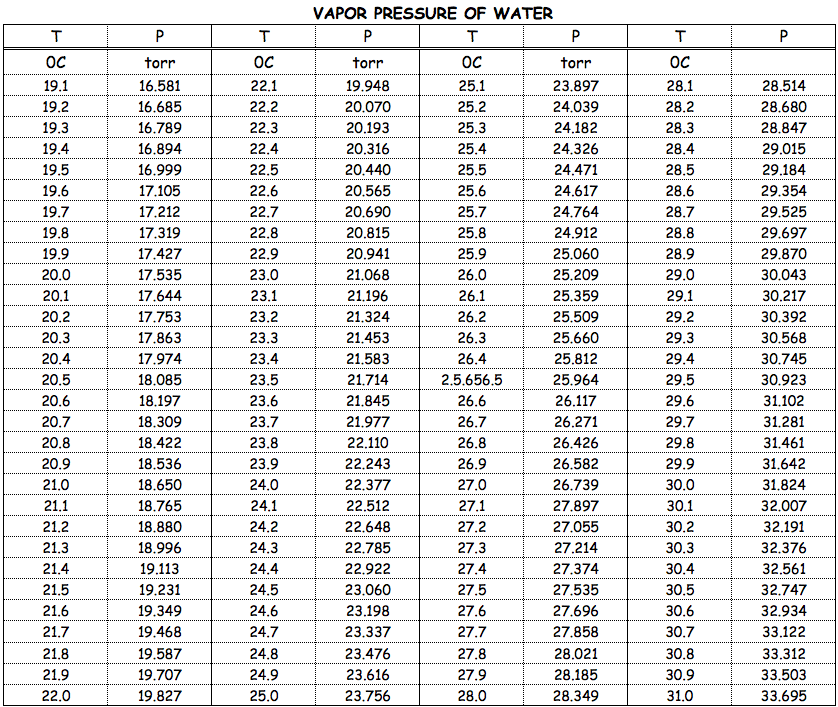
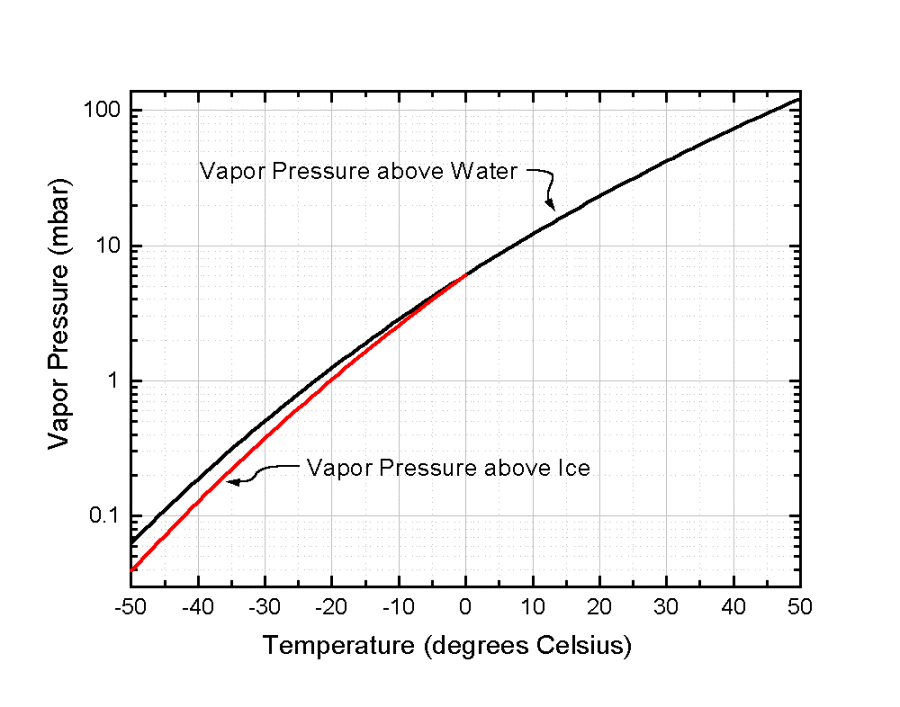
Vapor pressure of water at room temperature mmhg. Water temperature in celsius a b c. If you want the saturated vapor pressure enter the air temperature. If you want the actual vapor pressure enter the dewpoint. As for other substances water vapour pressure is a function of temperature and can be determined with.
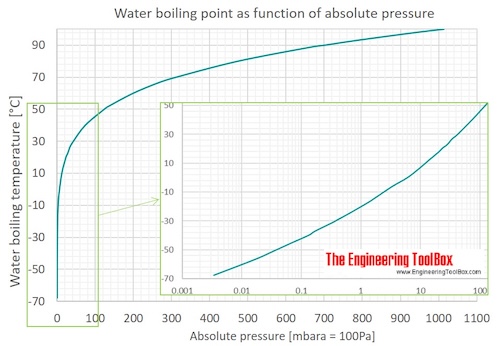
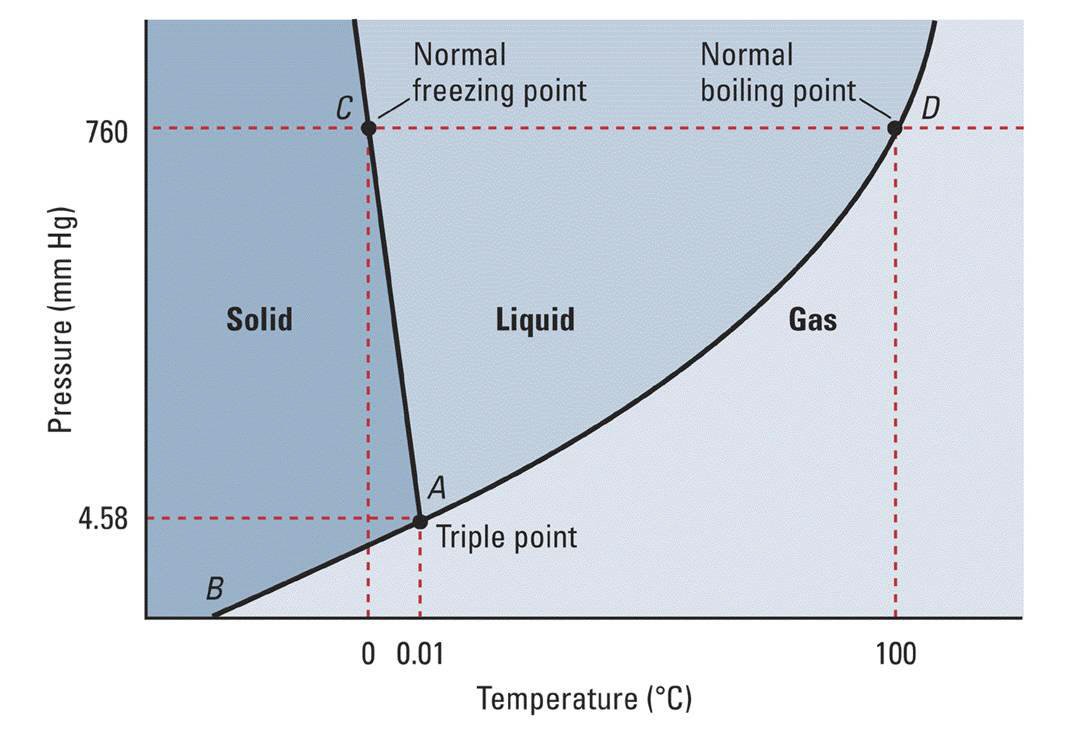
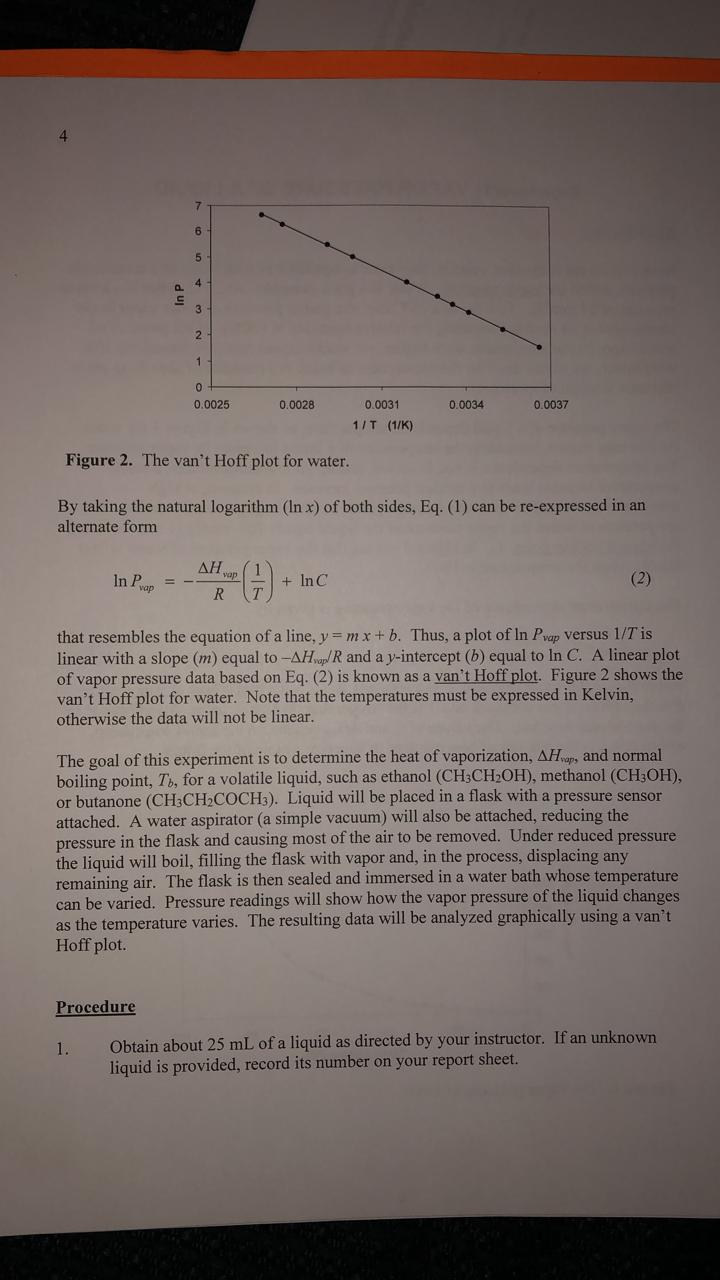
Water will have a vapor pressure of. This is therefore the boiling point of water at 1000 mmhg. The vapour pressure of water is the pressure at which water vapour is in thermodynamic equilibrium with its condensed state at higher pressures water would condense the water vapour pressure is the partial pressure of water vapour in any gas mixture in equilibrium with solid or liquid water. When the temperature in the range of 99 374 degree celsius a 8 14019 b 1810 94 and c 244 485.
Mb in of hg mm hg hpa kpa lbs per square in. See spelling differences or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases solid or liquid at a given temperature in a closed system the equilibrium vapor pressure is an indication of a liquid s evaporation rate. Mb in of hg mm hg. It relates to the tendency of particles to.
A the vapor pressure curve of water intersects the p 1000 mmhg line at about 110 c. Pressure degrees c mmhg degrees c mmhg. Assume we want to calculate the vapor pressure of water in 86 f 30 c. 1 torr 1 mm hg one millimeter of mercury.
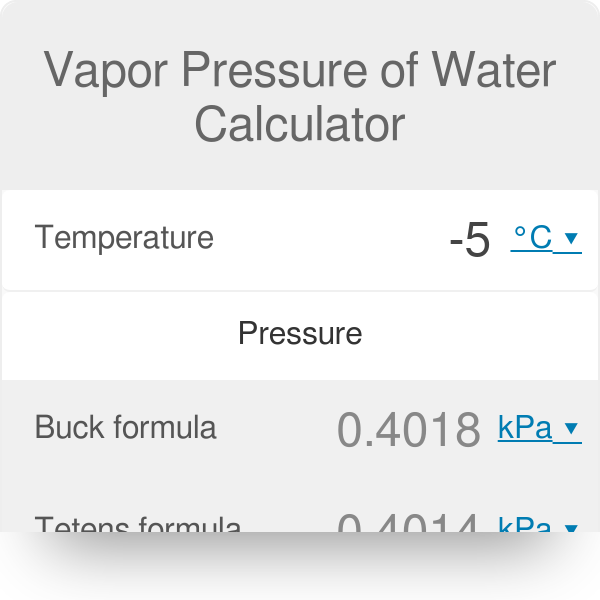
But remember that vapor pressures increase with temperature. P 10 a b c t where. The most often used is the antoine equation 4 232 kpa but the buck formula 4 245 kpa is usually the most accurate one for temperature ranges we typically look for. Enter a temperature or a dewpoint or both.
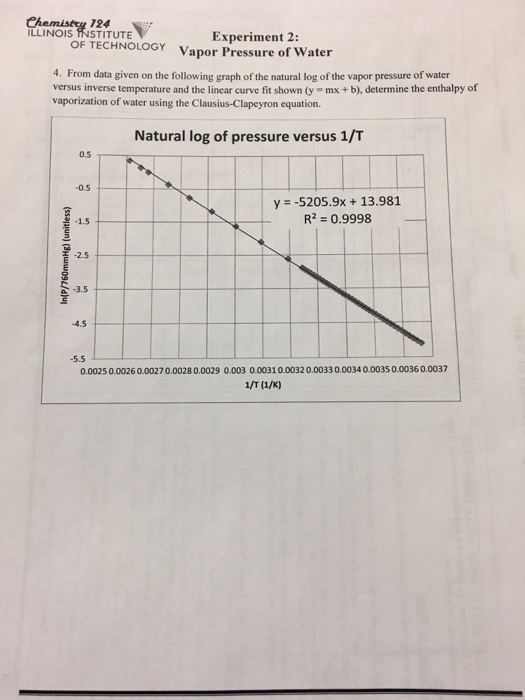
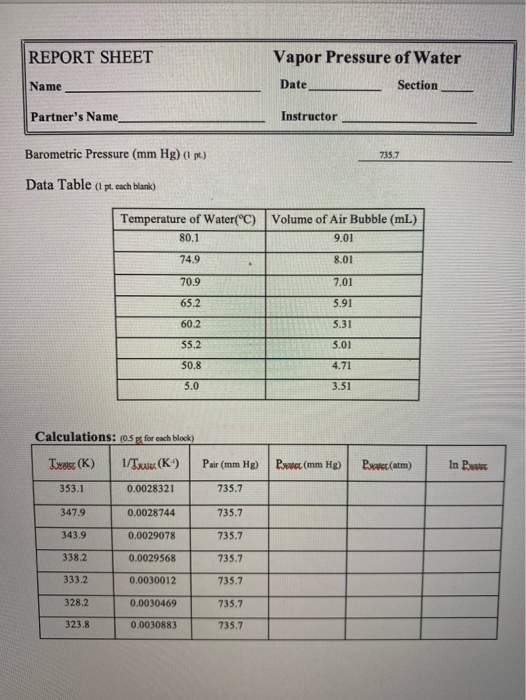
Vapor pressure of water. The vapor pressure of water calculator found the pressure according to five formulas. Vapor pressure of water t. For example water has a vapor pressure of approximately 20 torr at room temperature 22 c 72 f.
The most common unit for vapor pressure is the torr. Antoine constants for water when water temperature in the range of 1 100 celsius a 8 07131 b 1730 63 c 233 426. Most materials have very low vapor pressures. The temperature of the solution is 25 c and the vapor pressures of each of these chemicals at 25 c is 95 1 mm hg for benzene 28 4 mm hg for toluene.